LociOiling (talk | contribs) (Clarify missing cysteine and methionine.) |
LociOiling (talk | contribs) (Link to polar.) |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | The |
+ | The images in this gallery show the amino acid [[Sidechain|sidechains]] with atoms that can form [[Hydrogen bond|hydrogen bonds]]. These sidechains are marked as [[Polar|polar]] in the table of [[Amino Acids|amino acids]]. |
| + | Blue arrows show atoms which can act as hydrogen bond donors. Red arrows show atoms which can act as hydrogen bond acceptors. Generally, red oxygen atoms are acceptors, and blue nitrogen atoms are donors, but there are exceptions, depending of the presence of hydrogen. |
||
| ⚫ | |||
| − | |||
| ⚫ | |||
| − | |||
| ⚫ | The |
||
| − | |||
| − | Blue arrows show possible hydrogen bond donations (one per hydrogen atom). Red arrows show where hydrogen bonds can be accepted. |
||
| − | |||
| − | Black arrows and numbers show the bondable heavy atom. (In the last segment of a protein section, known as the [[C terminal]], add one to the atom number shown.) The atom numbers can be used when creating [[Rubber Bands|bands]] in a Foldit [[Recipes|recipe]]. The [[Sidechain Bonding Table]] summarizes the bondable atoms. |
||
| − | |||
| ⚫ | |||
| − | |||
| ⚫ | Note that the two sulfur-containing sidechains, [[Cysteine|cysteine]] and [[Methionine|methionine]] aren't included here. The sulfur in methionine doesn't form additional bonds, while the sulfur at the tip of cysteine can form a [[Disulfide Bridge|disulfide bridge]], which is type of [[Covalent bond|covalent bond]]. Covalent bonds are much stronger than hydrogen bonds. |
||
| + | The atom number of each bondable atom is shown. See the "Details" section below for more. |
||
<gallery navigation="true" hideaddbutton="true"> |
<gallery navigation="true" hideaddbutton="true"> |
||
serine.bonding.png|Serine.|link=Serine |
serine.bonding.png|Serine.|link=Serine |
||
| − | threonine.bonding.png| |
+ | threonine.bonding.png|Threonine.|link=Threonine |
glutamate.bonding.png|Glutamate (glutamic acid).|link=Glutamate |
glutamate.bonding.png|Glutamate (glutamic acid).|link=Glutamate |
||
aspartate.bonding.png|Aspartate (aspartic acid).|link=Aspartate |
aspartate.bonding.png|Aspartate (aspartic acid).|link=Aspartate |
||
glutamine.bonding.png|Glutamine.|link=Glutamine |
glutamine.bonding.png|Glutamine.|link=Glutamine |
||
asparagine.bonding.png|Asparagine.|link=Asparagine |
asparagine.bonding.png|Asparagine.|link=Asparagine |
||
| − | histidine.bonding.png|Histidine.|link=Histidine |
+ | histidine.bonding.png|Histidine, first [[Tautomer|tautomer]], see below.|link=Histidine |
| + | Histidine.tautomer2.bonding.png|Histidine, second [[Tautomer|tautomer]], see below.|link=Histidine |
||
lysine.bonding.png|Lysine.|link=Lysine |
lysine.bonding.png|Lysine.|link=Lysine |
||
arginine.bonding.png|Arginine.|link=Arginine |
arginine.bonding.png|Arginine.|link=Arginine |
||
| − | tryptophan.bonding.png|Tryptophan |
+ | tryptophan.bonding.png|Tryptophan.|link=Tryptophan |
tyrosine.bonding.png|Tyrosine.|link=Tyrosine |
tyrosine.bonding.png|Tyrosine.|link=Tyrosine |
||
</gallery> |
</gallery> |
||
| + | |||
| + | ==Details== |
||
| + | The [[Amino Acid Gallery]] shows all 20 [[Amino Acids|amino acids]] used in Foldit. |
||
| + | |||
| ⚫ | |||
| + | |||
| ⚫ | |||
| + | |||
| + | Usually, nitrogen is a donor, but as the gallery shows, one of the nitrogens in [[Histidine|histidine]] acts as an acceptor. |
||
| + | |||
| + | Oxygen is either an acceptor, or a acceptor/donor, as in [[Serine|serine]], [[Threonine|threonine]], and [[Tyrosine|tyrosine]]. |
||
| + | |||
| + | As the diagram shows, hydrogen bonds are actually donated by hydrogen atoms attached to nitrogen or oxygen. Each hydrogen atom can contribute to one hydrogen bond. |
||
| + | |||
| ⚫ | |||
| + | |||
| + | The atoms numbers in this gallery are for the middle segments of a protein chain. In the last segment of a protein chain, known as the [[C terminal]], add one to the atom number shown. (Most Foldit puzzles contain only one protein chain, so the C terminal is the last segment. Very rarely, puzzles have more than one chain, so more than one C terminal.) |
||
| + | |||
| + | The atom numbers can be used when creating [[Rubber Bands|bands]] in a Foldit [[Recipes|recipe]]. |
||
| + | |||
| + | The [[Sidechain Bonding Table]] summarizes the bondable atoms. |
||
| + | |||
| + | [[Histidine]] is a special case, with two [[Tautomer|tautomers]]. In one tautomer, atom 10 is a donor, and atom 7 is an acceptor. In the other tautomer, the roles are reversed. Both atom 7 and 10 are nitrogen. The difference is which nitrogen has a hydrogen attached. See [http://fold.it/portal/node/2005025 Are all Histidines in Foldit the same?] for more detail. |
||
| + | |||
| ⚫ | |||
| + | |||
| ⚫ | Note that the two sulfur-containing sidechains, [[Cysteine|cysteine]] and [[Methionine|methionine]], aren't included here. The sulfur in methionine doesn't form additional bonds, while the sulfur at the tip of cysteine can form a [[Disulfide Bridge|disulfide bridge]], which is type of [[Covalent bond|covalent bond]]. Covalent bonds are much stronger than hydrogen bonds. |
||
| + | [[Category:Biochemistry]] |
||
Latest revision as of 22:38, 28 February 2020
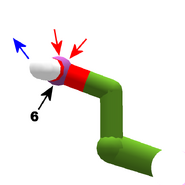
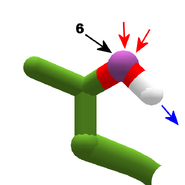
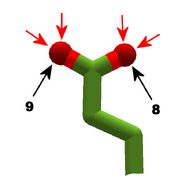
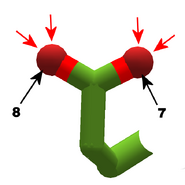
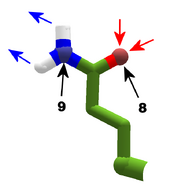
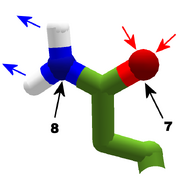
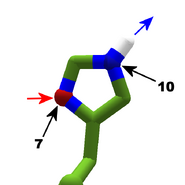
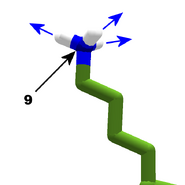
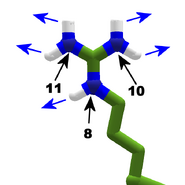
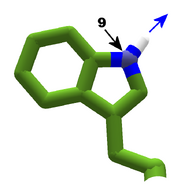
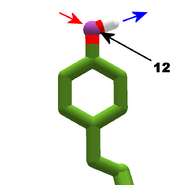
The images in this gallery show the amino acid sidechains with atoms that can form hydrogen bonds. These sidechains are marked as polar in the table of amino acids.
Blue arrows show atoms which can act as hydrogen bond donors. Red arrows show atoms which can act as hydrogen bond acceptors. Generally, red oxygen atoms are acceptors, and blue nitrogen atoms are donors, but there are exceptions, depending of the presence of hydrogen.
The atom number of each bondable atom is shown. See the "Details" section below for more.
Details[]
The Amino Acid Gallery shows all 20 amino acids used in Foldit.
Many amino acid sidechains contain oxygen and nitrogen atoms that can act as hydrogen bond donors or acceptors.
In View Options, the "show bondable atoms" option, combined with "show bonds (sidechain)", highlights the donors with blue spheres, and acceptors with red spheres. Some atoms can act as either a donor or acceptor, which is shown with a purple sphere.
Usually, nitrogen is a donor, but as the gallery shows, one of the nitrogens in histidine acts as an acceptor.
Oxygen is either an acceptor, or a acceptor/donor, as in serine, threonine, and tyrosine.
As the diagram shows, hydrogen bonds are actually donated by hydrogen atoms attached to nitrogen or oxygen. Each hydrogen atom can contribute to one hydrogen bond.
The view in this gallery is a combination of "cartoon thin" and "stick plus polar H". The "polar H" option reveals the hydrogen atoms, which normally aren't shown. In this view, hydrogens appear as white "caps" near a blue nitrogen or a red oxygen atom.
The atoms numbers in this gallery are for the middle segments of a protein chain. In the last segment of a protein chain, known as the C terminal, add one to the atom number shown. (Most Foldit puzzles contain only one protein chain, so the C terminal is the last segment. Very rarely, puzzles have more than one chain, so more than one C terminal.)
The atom numbers can be used when creating bands in a Foldit recipe.
The Sidechain Bonding Table summarizes the bondable atoms.
Histidine is a special case, with two tautomers. In one tautomer, atom 10 is a donor, and atom 7 is an acceptor. In the other tautomer, the roles are reversed. Both atom 7 and 10 are nitrogen. The difference is which nitrogen has a hydrogen attached. See Are all Histidines in Foldit the same? for more detail.
This gallery is based on this hydrogen bonding diagram, from Birkbeck College's online Principles of Protein Structures.
Note that the two sulfur-containing sidechains, cysteine and methionine, aren't included here. The sulfur in methionine doesn't form additional bonds, while the sulfur at the tip of cysteine can form a disulfide bridge, which is type of covalent bond. Covalent bonds are much stronger than hydrogen bonds.