No edit summary |
LociOiling (talk | contribs) (Freshen up a bit, fix grievous error.) |
||
| (6 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Helices_Horizontal.png|thumb|400px|Helixes (AAColor coloring, "cartoon thin" view). ]] |
||
| − | The helix is one of three basic structure types used in FoldIt. In its ideal form, it appears like a coiled cylindrical spring with all [[sidechain]]s directed outward. Ideal helices usually have hydrogen bonds between every third [[amino acid]] in the chain; this bonding is not visible in the helix representation, but it can be informative to change the helix to [[sheet]]s to see the hydrogen bond pattern.[[File:Helix.jpg|thumb|helix]] |
||
| + | [[Helix]] is one of three types of [[Secondary Structure|secondary structure]] in Foldit. The other types are [[Sheet|sheet]] and [[Loop|loop]]. |
||
| + | In its ideal form, a helix appears like a coiled cylindrical spring with its [[Sidechain|sidechains]] pointing outward. Ideal helixes usually have hydrogen bonds between every fourth [[Amino Acids|amino acid]] in the helix. |
||
| − | The helix structure is used to represent the real protein structure called an alpha helix. An ideal alpha helix will have one coil for every 3.6 amino acids. The closer a particular series of amino acids in your FoldIt puzzle is to that ratio, the more helpful it may be to define it as helix using [[Structure Mode]]. |
||
| + | Some times the prefix "alpha" is added, so you might see "alpha helix" or "α-helix". The "alpha" reflects the fact that helixes were the first type of protein structure discovered. |
||
| ⚫ | Certain amino acids have a tendency to form |
||
| + | The Foldit [[View Options|view options]] "Show Bonds" (basic GUI) or "Show Bonds (helix" (advanced GUI) can be used to visualize helix bonds. In the advanced GUI, the "cartoon" or "cartoon thin" protein view options show helixes with the coiled shape seen in the example on this page. |
||
| ⚫ | |||
| + | An ideal helix has one turn of the coil for every 3.6 amino acids. |
||
| ⚫ | Coiled-coil α |
||
| ⚫ | Certain amino acids have a tendency to form helixes, while others tend not to. Those that often (but not always) form helixes are [[Methionine|methionine]], [[Alanine|alanine]], [[Leucine|leucine]], uncharged [[Glutamic acid|glutamate]], and [[Lysine|lysine]] ("[[Methionine|M]][[Alanine|A]][[Leucine|L]][[Glutamic acid|E]][[Lysine|K]]" in the amino-acid 1-letter codes). Those that do not easily form helixes include [[Proline|proline]], [[Glycine|glycine]], and [[Aspartic acid|aspartate]]. Proline, however, may be found at the beginning of a helix. |
||
| ⚫ | |||
| ⚫ | |||
| − | <span class="author-p-10405 b" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">'''- '''</span><span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">AAs f</span><span class="author-p-10495" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">ound most in alpha</span><span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;"> helix are</span><span class="author-p-10495" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;"> glutamate, methionine, alanine, and leucine.</span> |
||
| + | ''Editor's note: this section is interesting, but it's perhaps a bit on the technical side for most.'' |
||
| − | <span class="author-p-10405 b" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">'''The '''</span><span class="author-p-10405 b i" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">'''''Alpha Helix'''''</span><span class="author-p-10405 b" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">''' structure'''</span><span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">:</span>*<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Rigid</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Rod-like structure</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">3.6 amino per turn</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Hydrogen bond (N-H- - -O=C)</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">4 residue ahead (n+4)</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Right-handed</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">1.5 Å translation</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">100 degrees</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Pitch: 54 degree</span> |
||
| − | *<span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Average size between 40 to +1000 Å (100nm or 0.1um)</span> |
||
| − | <span class="author-p-10405 b" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">'''A Helix with a twist, right or left?'''</span> |
||
| ⚫ | Coiled-coil α helixes are highly stable forms in which two or more helixes wrap around each other in a "supercoil" structure. Coiled coils contain a highly characteristic sequence motif known as a heptad repeat, in which the motif repeats itself every seven residues along the sequence. The first and especially the fourth residues (known as the a and d positions) are almost always hydrophobic (the fourth residue is typically leucine) and pack together in the interior of the helix bundle. In general, the fifth and seventh residues (the e and g positions) have opposing charges and form a salt bridge stabilized by electrostatic interactions. Fibrous proteins such as keratin and myosin often adopt coiled-coil structures, as do several dimerizing proteins. A pair of coiled-coils - a four-helix bundle - is a very common structural motif in proteins. For example, it occurs in human growth hormone and several varieties of cytochrome. The Rop protein, which promotes plasmid replication in bacteria, is an interesting case in which a single polypeptide forms a coiled-coil and two monomers assemble to form a four-helix bundle. |
||
| − | <span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">When you look at a helix from the end and the top twist to the left it is a '''"S" left hand twist''' helix. A '''"Z" right hand''' twist helix well twist to the right at the top when viewed from the end. A "Z" twist helix is happy helix, and a "S" twist helix is a very un-happy helix.</span> |
||
| ⚫ | |||
| − | <span class="author-p-10405" style="padding-top: 0px; padding-bottom: 1px; cursor: auto;">Rope and twine making include both '''"Z" right hand''' twist strands and a gentler '''"S" left hand twist '''to all the bundles of those strands. Both "Z" and "S" twists are essential to stablize the torsional forces produced on a rope when it is stretched. This is the same when forming biological assemblies into larger strands. So the smaller strands are twisted one way, usualy "Z", and the bigger bundles are twisted more gently the other "S" way . Ropes need both "S" and "Z" twists to be stable otherwise if all the strands in a rope or biological structure were all "S" or "Z" twisted, the rope or strand will simply fall apart when pulled on. DNA is triple twisted into very tight compact strands. However unlike biological structures lightbulb filliments are triple twisted helixes, but being made of a flat ribbon of tungstin metal all the helix twist the same way around. </span> |
||
[[Category:Protein Structure]] |
[[Category:Protein Structure]] |
||
| + | [[Category:Glossary]] |
||
Latest revision as of 21:50, 26 January 2018
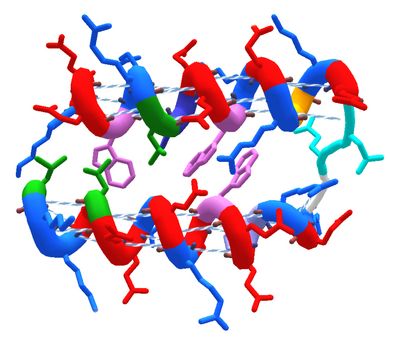
Helixes (AAColor coloring, "cartoon thin" view).
Helix is one of three types of secondary structure in Foldit. The other types are sheet and loop.
In its ideal form, a helix appears like a coiled cylindrical spring with its sidechains pointing outward. Ideal helixes usually have hydrogen bonds between every fourth amino acid in the helix.
Some times the prefix "alpha" is added, so you might see "alpha helix" or "α-helix". The "alpha" reflects the fact that helixes were the first type of protein structure discovered.
The Foldit view options "Show Bonds" (basic GUI) or "Show Bonds (helix" (advanced GUI) can be used to visualize helix bonds. In the advanced GUI, the "cartoon" or "cartoon thin" protein view options show helixes with the coiled shape seen in the example on this page.
An ideal helix has one turn of the coil for every 3.6 amino acids.
Certain amino acids have a tendency to form helixes, while others tend not to. Those that often (but not always) form helixes are methionine, alanine, leucine, uncharged glutamate, and lysine ("MALEK" in the amino-acid 1-letter codes). Those that do not easily form helixes include proline, glycine, and aspartate. Proline, however, may be found at the beginning of a helix.
Helixes as Part of Larger Structures[]
Editor's note: this section is interesting, but it's perhaps a bit on the technical side for most.
Coiled-coil α helixes are highly stable forms in which two or more helixes wrap around each other in a "supercoil" structure. Coiled coils contain a highly characteristic sequence motif known as a heptad repeat, in which the motif repeats itself every seven residues along the sequence. The first and especially the fourth residues (known as the a and d positions) are almost always hydrophobic (the fourth residue is typically leucine) and pack together in the interior of the helix bundle. In general, the fifth and seventh residues (the e and g positions) have opposing charges and form a salt bridge stabilized by electrostatic interactions. Fibrous proteins such as keratin and myosin often adopt coiled-coil structures, as do several dimerizing proteins. A pair of coiled-coils - a four-helix bundle - is a very common structural motif in proteins. For example, it occurs in human growth hormone and several varieties of cytochrome. The Rop protein, which promotes plasmid replication in bacteria, is an interesting case in which a single polypeptide forms a coiled-coil and two monomers assemble to form a four-helix bundle.