
The Koga & Koga paper as seen in Pubmed.
Koga & Koga describes promising secondary structure patterns for designing new proteins.
Koga & Koga includes analysis of both low-level patterns for connecting helixes and sheets and high-level structures based on complete natural proteins.
See Design Structures for a summary of the low-level patterns in Koga & Koga. These patterns are similar to the shapes found in the Blueprint tool in Foldit.
The low-level patterns rely on a discussion of left or right "chirality" or handedness for patterns involving two sheets connected by a short section of loop. For patterns involving a helix and a sheet connected by loop, Koga & Koga discusses "parallel" versus "anti-parallel" alignment.
Koga & Koga has precise mathematical definitions for left or right chirality and parallel or anti-parallel alignment.
The 3D atom coordinates used to calculate chirality and alignment aren't available in Foldit. Instead Design Structures show how to determine these properties by looking at the protein in Foldit.
This page examines the technical language in Koga & Koga in more detail, from a Foldit point of view. It looks at the Koga & Koga shortcuts involving the "pleats" of the sheet section of the patterns. The pleat method is equivalent to the sidechain method presented in Design Structures.
In all of these patterns, order matters. Sheet-loop-helix is not the same as helix-loop-sheet. The structure mentioned first always has the lower segment numbers. In the images on this page, blue arrows indicate increasing segment numbers.
alpha and beta[]
Koga & Koga uses the Greek letter α (alpha) for helix and the Greek letter ß (beta) for sheet.
So when the paper talks about "ß-hairpins", it's referring to short sections of loop that make a hairpin turn between two sheets.
These Greek letters reflect the fact that helixes were the first secondary structure identified in the early days of X-ray crystallography, so they became "alpha". Sheets were next, so they became "beta".
sheets and strands[]
Koga & Koga talks about "strands" where Foldit generally uses "sheets". Technically, sheets are formed when two or more strands form hydrogen bonds.
chirality[]

Sheets with left chirality are most common, except when there are five loop segments. The last "pleat" of the first sheet points toward the other sheet.
Koga & Koga refers chirality, or "handedness", which is important for the sheet-loop-sheet patterns. Chirality is either left (L) or right (R). There's a precise mathematical definition, but also a shortcut that's easy to understand in Foldit.
In the "cartoon" view in Foldit, the direction of the last "pleat" of the first sheet indicates whether chirality is L or R. If the pleat points toward the other sheet, the two sheets have left chirality, otherwise it's right chirality.
If you turn on "show bonds (sheet)" and "show bondable atoms", the last pleat is usually indicated by a red sphere, indicating a hydrogen bond acceptor. (In some cases, the red sphere seems to get buried inside the backbone, but the corresponding oxygen atom is still there.)
Design Structures uses the direction of end sidechains in a sheet-loop-sheet pattern, which amounts to the same thing as looking at the pleats.
Looking at pleats is also helpful for understanding the patterns involving a sheet and a helix, as described below.
parallel and anti-parallel[]

Parallel helix and sheet. The last pleat on the sheet points toward the helix. Most common with two loop segments.
For patterns involving a helix and a sheet, Koga & Koga uses "parallel" (P) and "anti-parallel" (A) to describe different alignments.
Once again, there's a precise mathematical description of these terms, but there's also a shortcut involving pleats and sheets.
For either the sheet-loop-helix case or the helix-loop-sheet case, the pleat of the sheet nearest the loop determines parallel versus anti-parallel.
For sheet-loop-helix, the alignment is parallel if the last sheet pleat points toward the helix. Otherwise, the sheet and the helix are anti-parallel.
For helix-loop-sheet, the alignment is parallel if the first sheet pleat points away from the helix. Otherwise, it's anti-parallel.
Rules[]
Koga and Koga offers three rules, again using the Greek letters for helix and sheet. The rules could be summarized as:
- ßß rule: sheet to sheet, chirality matters
- ßα rule: sheet to helix, parallel versus anti-parallel matters
- αß rule: helix to sheet, parallel versus anti-parallel matters, but parallel usually wins
sheet-loop-sheet: the ßß rule[]

Sheets with right chirality are more common with five loop segments. The last "pleat" of the first sheet points away from the other sheet.
The ßß rule involves a pattern where two sheets (or strands) are connected by a short section of loop.
For this pattern, the number of segments in the loop determines whether left (L) or right (R) chirality is preferred.
With two loops, left chirality is found in almost all natural proteins with this pattern.
With three loops, left chirality is still more common, but not to the extent seen for 2 loops.
With four loops, natural proteins are closely divided between left and right chirality.
With five loops between the sheets, natural proteins are more likely to show right chirality.
sheet-loop-helix: the ßα-rule[]

Anti-parallel sheet and helix. The last pleat of the sheet points away from the helix. Most common with three loop segments.
This pattern involves a sheet (or beta-strand) connected to a helix by a short section of loop.
Here, Koga & Koga identifies parallel versus anti-parallel alignment as the key factor. Again, looking at pleats makes it easy to determine which is which.
If the pleat of the last segment of the sheet points toward the helix, it's a parallel (P) alignment. If the pleat points away from the helix, it's anti-parallel (A).
When there are two loops between the sheet and the helix, parallel alignment is much more common than anti-parallel in natural proteins.
When there are three loops between the sheet and the helix, anti-parallel alignment becomes more common.
Koga & Koga didn't include a rule for longer sections of loop between sheet and helix.
helix-loop-sheet: the αß rule[]
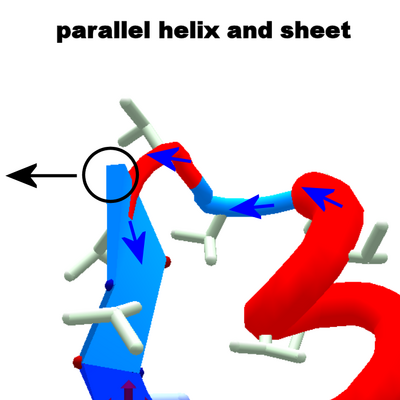
Parallel helix and sheet. The first pleat of the sheet points away from the helix. (The red dot normally seen on the pleat is hidden.) This arrangement is most common regardless of loop length.
The "αß rule" covers cases involving a helix connected to a sheet by a short section of loop,.
Once again, the pleat of the sheet indicates parallel versus anti-parallel. Since the helix comes first in this case, the first pleat of the first segment of the sheet indicates direction. If the pleat points away from the helix, it's a parallel (P) alignment, otherwise, it's anti-parallel (A).
The Koga & Koga results show a strong preference for parallel orientation for loops with length 2, 3, or 4. For "native" or natural proteins, there are some anti-parallel examples. For example, at loop length 4, there are about 600 anti-parallel cases, but over 1,200 parallel cases.